At ADPD, Scientists Dissect the Ins and Outs of Tau Propagation
Quick Links
The revelation that tau aggregates can pass between cells, corrupting their intracellular counterparts, changed how the Alzheimer’s field studies tau pathology. At this year’s AD/PD meeting, held in March 5-9 in Lisbon, Portugal, researchers focused less on how the microtubule-binding protein travels from place to place, and more on what happens after it arrives. For example, they presented evidence that an ATPase—valosin containing protein—plays decisive roles in dismantling, and amplifying, incoming tau seeds in the cytoplasm. Others described how intracellular tau seeding takes place within astrocytes, and that blocking the release of extracellular vesicles, including those carrying tau, curbs tau pathology in the mouse brain. Further evidence implicated tau oligomers, rather than tangles, in neuronal demise. The focus on the intracellular mechanisms underlying tau propagation comes at a time when preclinical and clinical drugs’ discovery efforts increasingly focus on intracellular tau (see Part 3 of this series).
- Valosin-containing protein can either help or hinder intracellular tau seeding.
- Astrocyte biosensor cell lines reveal unique intracellular seeding patterns.
- Shutting down extracellular vesicle release halts the propagation of tau pathology in mice.
- Neurons on the precipice of death rarely have tau tangles.
Seed Fate Hinges on VCP
A decade has passed since Marc Diamond of UT Southwestern in Dallas generated the now widely used tau biosensor cell lines, which detect intracellular tau seeding via FRET signals emitted when two molecules of tau snap together (Oct 2014 news). Since then, Diamond and other researchers have used these HEK293 cells, along with mouse models, to investigate the mechanisms involved in the seeding and propagation of tau pathology. Most recently, his lab reported that tau aggregates spread retrogradely—from post- to presynapses —between neurons (Jun 2023 news).
In Lisbon, Diamond focused on the intracellular events that go on after tau passes from one cell to another. He noted that most internalized tau aggregates are destined for degradation in the endolysosomal system. However, according to recent work from the lab, a small proportion of tau escapes the confines of these digestive vesicles and crosses into the cytosol, where they corrupt endogenous tau (Dodd et al., 2022). Once set in motion, the seeds replicate in the cytoplasm with incredible efficiency, he said. “The question of the day is, what is the cellular machinery that allows this to happen?”
To answer this, postdoc Sushobhna Batra and colleagues ran a screen for proteins that greet tau fibrils upon their arrival in the cytoplasm. They used the so-called split APEX2 system developed by Alice Ting at Stanford University. Essentially, instead of using FRET to detect tau aggregation, this system brings together two parts of a peroxidase enzyme when two units of tau join. Then, by adding biotin-phenol along with peroxide, all proteins in proximity to the tau pair become biotinylated, allowing the researchers to isolate and identify them. This way, Batra hunted for proteins associated with the earliest stage of tau seeding, at five hours after fibril exposure. The top hit, by several orders of magnitude, was valosin containing protein (VCP). The AAA+ ATPase supports a multitude of cellular processes. Chief among them is the plucking of individual proteins from membranes or larger complexes for the purpose of refolding, recycling, or disposal. The protein has also been tied to neurodegenerative tauopathies, and just last year, Diamond, in collaboration with Ulrich Hartl of the Max Planck Institute of Biochemistry, nabbed VCP as a protein that disaggregates tau fibrils (Saha et al., 2023; Apr 2023 conference news).
How might this disaggregase influence tau seeding? In biosensor cell lines, Batra found that lowering VCP expression cut tau seeding efficiency by half. Treatment with the VCP inhibitor NMS-873 had a similar effect. Surprisingly, ML240, a different VCP inhibitor, did the opposite. It skyrocketed seeding from 2 to 3 percent of cells, to 95 percent of cells. Neither of these inhibitors had any effect on tau seeding if given eight or more hours after seed exposure, implying that VCP plays a role in the earliest stages of seeding. The inhibitors had similar effects in iPSC-derived neurons expressing the tau biosensor constructs.
What explains the opposing effects of the VCP inhibitors? A potential explanation emerged when Batra methodically knocked down each of the 30 known VCP cofactors in HEK293 biosensor cells. While most had no effect on seeding, one, called FAF2, increased seeding by 30 to 40 percent when knocked down, while six others did the opposite. Diamond proposed a model whereby some cofactors help VCP yank tau monomers from the end of a growing fibril, relegating them to the proteasome for degradation and slowing seeding. In contrast, other VCP cofactors might help the enzyme pluck tau monomers from the middle of a fibril, thus splitting the fibril in half and doubling the number of seeds, effectively accelerating seeding (image below). Diamond suggested that perhaps NMS-873 inhibits the seed-promoting VCP cofactors, while ML240 hinders the enzyme’s seed-stopping partners.

Seed Model. When tau seeds enter the cytoplasm, they encounter VCP. In this model, depending on which cofactors VCP is associated with, the ATPase removes monomers of tau from the end of the fibrils (bottom half), leading to fewer seeds, or from the middle of fibrils (top half), leading to more seeds. [Courtesy of Marc Diamond, UT Southwestern.]
Together, the inhibitor and knockdown findings paint VCP as a nexus that determines the fate of tau seeding, Diamond said. While the processes that govern expression of different VCP cofactors remain a mystery, Diamond told Alzforum that cofactor availability could contribute to selective vulnerability of some neurons to tau pathology. As such, Diamond believes VCP cofactors could make good druggable targets. What’s more, ongoing work in his lab suggests this VCP-related mechanism might be afoot not only for propagation of tau, but also for other proteopathic proteins, such as α-synuclein and TDP-43. He noted that James Shorter of the University of Pennsylvania in Philadelphia previously uncovered a similar relationship between Hsp104—a yeast AAA+ATPase—and the seeding of prion and prion-like proteins, and between nuclear transportins and seeding of RNA-binding proteins such as FUS in animals (Aug 2014 news; May 2017 news).
Astrocytes as Tau Seed Incubators
Neurons aren’t the only cells that deal with the menace of tau aggregates. Astrocytes have been found to shoulder the lion’s share of tau pathology in several neurodegenerative diseases, particularly in 4R tauopathies such as progressive supranuclear palsy, aging related astrogliopathy (ARTAG), and even in AD, noted Aurélien Lathuilière of the University of Geneva (Kovacs et al., 2016; Oct 2023 news; Nov 2020 news). The glial cells also express known tau aggregate receptors, such as LRP1 and heparin sulfate proteoglycans, at higher levels than their neuronal counterparts. For these reasons and more, Lathuilière said, studying tau uptake, seeding, and aggregation within astrocytes is critical. To do this, he created an astrocyte version of Diamond’s biosensor cell lines. In the astrocytoma line CCF-STTG1, Lathuilière used a lentiviral vector to stably express P301L tau fragments fused with complementary FRET probes, which, as a postdoc in Bradley Hyman’s lab at Massachusetts General Hospital in Charlestown, he had previously modified to enhance sensitivity to seeds (Lathuilière et al., 2023).
In Lisbon, Lathuilière reported that these astrocyte biosensor cells readily internalized tau seeds from AD brain lysates. Using immunostaining to detect the internalized phospho-tau, he found the internalized material in a dotted pattern, suggesting it inhabited vesicles of some kind. Staining with LAMP1 indicated that while some of these tau-carrying compartments were lysosomes, many others were not. Lathuilière suggested they could be endosomes destined for lysosomal fusion. Next, Lathuilière measured seeding efficiency of the internalized seeds via FRET. He found that inhibiting lysosomal function with hydroxychloroquine dramatically enhanced seeding within astrocytes. Revving up metabolic stress by feeding the astrocytes fatty acids also enhanced tau seeding. Blocking the LRP1 receptor only dampened tau aggregation by 30 percent, suggesting that other receptors, in addition to LRP1, help internalize tau seeds.

Nuclear Tau? Tau aggregates associated with the nuclear membrane in a small proportion of astrocyte biosensor cells. [Courtesy of Aurélien Lathuilière, University of Geneva.]
Lathuilière was in for a surprise when he looked closer at the tau aggregates that formed within the biosensor astrocytes. While most of the visible inclusions resided in the cytoplasm, in about 10 percent of the cells, aggregates formed within the nucleus, where they associated with the nuclear membrane (image above). Tau has been spotted loitering in and around the nucleus before, including within nuclear speckles, which are hubs for the cell’s splicing machinery (Sep 2018 news; Jan 2019 news; Apr 2021 news). However, Lathuilière said that the pattern of tau’s association with the nucleus in the astrocyte biosensor cells appeared far more extensive compared to previous reports. Ongoing studies in his lab are focused on understanding this odd form of tau aggregation.
Finally, Lathuilière treated the astrocytes with high-molecular-weight tau oligomers extracted from human AD brain lysates, which he believes are the most potent form of tau seeds. He then looked for changes in their transcriptomes. When faced with tau seeds, the cells adopted a gene-expression profile much like astrocytes in the AD brain. This suggests that these astrocytoma cells approximate physiological responses to tau pathology, Lathuilière said.
Given the proposed endolysosomal localization of tau aggregates in the astrocytes, Doo Yeon Kim of Massachusetts General Hospital asked Lathuilière if he had investigated whether astrocytes might secrete tau seeds via exosomes, which derive from lysosomal compartments. Lathuilière said that his lab is studying if and how astrocytes secrete internalized tau seeds.
Previously, Roberto Piacentini of Università Cattolica del Sacro Cuore in Rome found that, relative to neurons, astrocytes have a voracious appetite for tau oligomers, which the cells internalize via glypican-4 (GPC4), a heparin sulfate proteoglycan. Once internalized, the oligomers bungle the release of gliotransmitters, which causes synaptic dysfunction in associated neurons (Dec 2018 conference news). In Lisbon, Piacentini reported that none other than the C-terminal domain of amyloid precursor protein, AICD, induces the expression of GPC4. Once cleaved from APP, AICD binds directly to the GPC4 promotor, Piacentini said. When astrocytes were deprived of APP, they downregulated GPC4 and lost their appetite for tau oligomers, curbing synaptotoxicity nearby. Piacentini reported some of this last year (Puliatti et al., 2023). His work suggests that when astrocytes take up tau oligomers directly, the cells sour neuronal function indirectly. No spewing of tau seeds required.
Stopping Tau in its Tracks
For better or worse, there is plenty of evidence that tau aggregates do travel, and that they ride in extracellular vesicles. Seiko Ikezu and Tsuneya Ikezu of the Mayo Clinic in Jacksonville, Florida, previously reported that tau trafficked to neurons via exosomes released from microglia (Oct 2015 news). The researchers later pegged the ATP-gated cation channel, P2X purinoceptor 7 (P2RX7), with triggering exosomal secretion in microglia, and reported that a P2RX7 inhibitor reduced tau accumulation and rescued memory loss in PS19 mice (Ruan et al., 2020). Later, Seiko Ikezu reported similar benefits could be had by deleting from microglia TSG101, a protein that forms extracellular vesicles (Apr 2023 conference news).
At AD/PD, she focused on P2RX7. Scientists in her lab have generated mice lacking the receptor, and have crossed them to PS19 tauopathy mice. At 9 months of age, the hippocampal region of PS19 controls is inundated with intracellular, hyperphosphorylated aggregates of tau. Deleting the cation channel substantially squashed this pathology, with one exception: the mossy fiber region, where P2RX7 knockouts had a higher burden of tau aggregates (image below). Ikezu does not have an explanation for this, but noted that mossy fibers typically have high expression of P2RX7. In a barrage of behavioral tests, deletion of P2RX7 restored the flagging learning and memory in PS19 mice to wild-type levels, suggesting an overall salubrious effect. It also prevented shrinkage of the cortex and hippocampus.
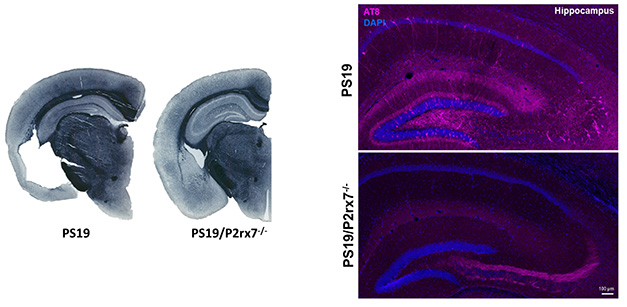
No P2RX7, No Problem. PS19 mice have profound brain shrinkage (left) and accumulation of hyperphosphorylated tau (AT8, pink) runs amok in several regions of the hippocampus (top right). Both problems are substantially reduced in PS19 mice lacking the P2RX7 cation channel (P2rx7-/-). [Courtesy of Seiko and Tsuneya Ikezu, Mayo Clinic.]
Did P2XR7 knockout influence the quality and quantity of extracellular vesicles released by cells in the brain? Ikezu has some preliminary findings on this. For one, cryo-EM analysis of EVs isolated from the mouse brain revealed that, compared to wild-type animals, PS19 mice had larger EVs, and more of them. Knocking out P2RX7 restored EV size and quantity back to wild-type levels. A proteomic analysis uncovered nearly 200 proteins that were more abundant in PS19 EVs relative to wild-type, but these were normalized by P2RX7 knockout. Tantalizingly, MAPT and ApoE were among these proteins, as were several involved in mitochondrial function. Finally, Ikezu expressed a fluorescently tagged EV marker—CD9—under control of either microglial or neuronal promoters, to assess how P2RX7 influenced EV release from both cell types. She reported a profound reduction in EV release from both, with the strongest effect in microglia. In ongoing work she is focused on deciphering which cell types and subtypes are responsible for P2RX7-mediated spread of tau pathology in EVs.
“How EVs secreted from each neuronal cell type affect each other is still a mystery, and many investigators, including in our own lab, are trying to shed light on intercellular communication by EVs,” Ikezu told Alzforum. She and colleagues are also developing P2RX7 inhibitors with an eye toward clinical development. In addition to carting tau aggregates, EVs also transport inflammatory molecules, such as cytokines, between cells, so it’s possible that inhibiting EV release with P2RX7 inhibitors might stem both tau propagation and inflammation, she said.
One attendee asked Ikezu whether blockage of EV secretion altered the overall state of microglia. Ikezu said that they are planning single-cell RNA-Sequencing studies to answer this question.
Invisible Tau and Shunned Neurons
Which forms of tau promote propagation of pathology across the brain, and which are most harmful to neurons? These questions were recurrent throughout this year’s AD/PD meeting, with several scientists taking the view that oligomers, as opposed to fibrils, play a leading role. Case in point, Hyman recently reported that both fibrillar and oligomeric tau are capable of seeding tau aggregation when injected into the PS19 mouse brain. However, he found that of the two, high-molecular-weight oligomers were far more adept at triggering widely disseminated tau aggregates (Mate de Gerando et al., 2023). In Lisbon, Hyman shifted gears away from seeding, and focused instead on another fundamental question: Which species of tau aggregates are neurotoxic? He noted that while correlations between tau tangles and neurodegeneration are rock-solid at the brain region level, some findings suggest that this correlation might not hold up at the level of individual cells. Hyman suspects that oligomeric tau, which he calls “the tau we cannot see,” could be more neurotoxic than bona fide tangles.

Dying Alone. Neighboring neurons appear to scoot away from a neuron on the precipice of death (center). [Courtesy of Bradley Hyman, Massachusetts General Hospital, Boston.]
To investigate, the Hyman lab used in vivo multiphoton imaging to track the growth of tangles, as well as the loss of individual neurons, over time. In both rTg4510 and Thy-tau22 mouse models, the researchers used a fixed camera to check in on the same neurons week after week. When a neuron turned up missing, it was presumed dead, and researchers could then refer back to images from earlier sessions to look for signs of that neuron’s impending demise. Using this technique, they found that, as expected, the number of tangle-bearing neurons increased week after week. Neuronal death was a rare event, but the researchers were able to document 64 incidents in rTg4510 mice over four weeks. Strikingly, they found that few doomed neurons had tangles prior to their passing. Rather, the rate of neuronal loss was threefold higher among neurons without tangles than it was for neurons with them. Hyman said the findings suggest that tangles are not a death sentence, and that their formation might even be a marker of resilience.
If not tau tangles, were there any other telltale signs of impending neuronal death? Indeed, Hyman and colleagues found that their closest neighbors appeared to ditch them at their darkest hour. Using three-dimensional longitudinal imaging, the researchers found that one to two weeks before a neuron disappeared, the space between the dying neuron and its nearest neighbors increased by up to 60 percent, creating a “hole” surrounding the ill-fated neuron. Hyman still doesn’t know how this happens, but he wondered if this morphological feature could help identify and study dying neurons in postmortem human brain samples. In support of that idea, in thick, cleared sections from AD brain samples, the researchers spotted these holes surrounding about 2 percent of neurons, roughly matching the calculated rate of neuronal loss in the AD brain. These loners rarely had tau tangles. In control brains, few if any of these deserted neurons were spotted. Hyman hopes to use this marker to study how tau tangles, and other characteristics, relate to neuronal death in the human brain.—Jessica Shugart
References
News Citations
- Therapeutic Contenders Target Hard-to-Reach Pockets of Tau
- Cellular Biosensor Detects Tau Seeds Long Before They Sprout Pathology
- Tau Propagation Surprise: It Might Travel Retrogradely
- Tau Chimeras Do Make Fibrils—and a Chaperone Rips Them Apart
- Yeast Chaperone Melts Protein Aggregates
- Protein Liquid-Liquid Phase Transitions: The Science Is About to Gel
- Tauopathy Transcriptomes Tell Tantalizing Tales
- Does Astrocyte Tau Cause Dementia?
- Tau Stymies Transport Through Neuron’s Nucleus
- Invasion of the Microtubules: Mutant Tau Deforms Neuronal Nuclei
- Tau, Speckle Wrecker, Disrupts the Nuclear Home
- Toxic Tau: Who Are You, and Where Are You From?
- Deadly Delivery: Microglia May Traffic Tau Via Exosomes
- From Phagocytosis to Exophagy: Microglia's Digestive Tract Dissected
Research Models Citations
Paper Citations
- Dodd DA, LaCroix M, Valdez C, Knox GM, Vega AR, Kumar A, Xing C, White CL, Diamond MI. Tau seeds translocate across the cell membrane to initiate aggregation. medRxiv, May 12, 2022
- Saha I, Yuste-Checa P, Da Silva Padilha M, Guo Q, Körner R, Holthusen H, Trinkaus VA, Dudanova I, Fernández-Busnadiego R, Baumeister W, Sanders DW, Gautam S, Diamond MI, Hartl FU, Hipp MS. The AAA+ chaperone VCP disaggregates Tau fibrils and generates aggregate seeds in a cellular system. Nat Commun. 2023 Feb 2;14(1):560. PubMed.
- Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, Cairns NJ, Crary JF, Duyckaerts C, Ghetti B, Halliday GM, Ironside JW, Love S, Mackenzie IR, Munoz DG, Murray ME, Nelson PT, Takahashi H, Trojanowski JQ, Ansorge O, Arzberger T, Baborie A, Beach TG, Bieniek KF, Bigio EH, Bodi I, Dugger BN, Feany M, Gelpi E, Gentleman SM, Giaccone G, Hatanpaa KJ, Heale R, Hof PR, Hofer M, Hortobágyi T, Jellinger K, Jicha GA, Ince P, Kofler J, Kövari E, Kril JJ, Mann DM, Matej R, McKee AC, McLean C, Milenkovic I, Montine TJ, Murayama S, Lee EB, Rahimi J, Rodriguez RD, Rozemüller A, Schneider JA, Schultz C, Seeley W, Seilhean D, Smith C, Tagliavini F, Takao M, Thal DR, Toledo JB, Tolnay M, Troncoso JC, Vinters HV, Weis S, Wharton SB, White CL 3rd, Wisniewski T, Woulfe JM, Yamada M, Dickson DW. Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol. 2016 Jan;131(1):87-102. Epub 2015 Dec 10 PubMed.
- Lathuiliere A, Jo Y, Perbet R, Donahue C, Commins C, Quittot N, Fan Z, Bennett RE, Hyman BT. Specific detection of tau seeding activity in Alzheimer's disease using rationally designed biosensor cells. Mol Neurodegener. 2023 Aug 8;18(1):53. PubMed.
- Puliatti G, Li Puma DD, Aceto G, Lazzarino G, Acquarone E, Mangione R, D'Adamio L, Ripoli C, Arancio O, Piacentini R, Grassi C. Intracellular accumulation of tau oligomers in astrocytes and their synaptotoxic action rely on Amyloid Precursor Protein Intracellular Domain-dependent expression of Glypican-4. Prog Neurobiol. 2023 Aug;227:102482. Epub 2023 Jun 14 PubMed.
- Ruan Z, Delpech JC, Venkatesan Kalavai S, Van Enoo AA, Hu J, Ikezu S, Ikezu T. P2RX7 inhibitor suppresses exosome secretion and disease phenotype in P301S tau transgenic mice. Mol Neurodegener. 2020 Aug 18;15(1):47. PubMed.
- Mate De Gerando A, Welikovitch LA, Khasnavis A, Commins C, Glynn C, Chun JE, Perbet R, Hyman BT. Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau. Acta Neuropathol. 2023 Aug;146(2):191-210. Epub 2023 Jun 21 PubMed.
Further Reading
Papers
- Stern AM, Selkoe DJ. Soluble oligomers or insoluble fibrils? Scientific commentary on "Tau seeding and spreading in vivo is supported by both AD-derived fibrillar and oligomeric tau". Acta Neuropathol. 2023 Dec;146(6):861-862. Epub 2023 Sep 21 PubMed.
- Mate de Gerando A, Quittot N, Frosch MP, Hyman BT. Reply: Soluble oligomers or insoluble fibrils?. Acta Neuropathol. 2023 Dec;146(6):863-866. Epub 2023 Sep 21 PubMed.
Annotate
To make an annotation you must Login or Register.

Comments
UT Southwestern Medical Center
A word of caution for those who are trying to extract biological meaning from tau RD biosensor cell lines: Nuclear tau aggregates are an artifact of the truncation used in the model. Disease-relevant full-length tau aggregates never localize to the nucleus in diverse experimental paradigms, a conclusion based on a decade of inquiry. Our cell lines were optimized to make predictions for aggregate burden etc, but are artifact-prone when assessing mechanisms of tau toxicity. Feel free to reach out to me for unpublished data and notes.
Make a Comment
To make a comment you must login or register.